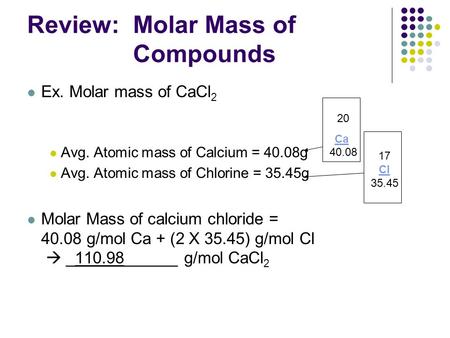
Mass Of Cl
- Chlorine (17 Cl) has 25 isotopes with mass numbers ranging from 28 Cl to 52 Cl and 2 isomers (34m Cl and 38m Cl). There are two stable isotopes, 35 Cl (75.77%) and 37 Cl (24.23%), giving chlorine a standard atomic weight of 35.45. The longest-lived radioactive isotope is 36 Cl, which has a half-life of 301,000 years. All other isotopes have.
- Use the atomic masses of each of the two isotopes of chlorine along with their percent abundances to calculate the average atomic mass of chlorine. Step 1: List the known and unknown quantities and plan the problem. Chlorine-35: atomic mass = 34.969 amu and% abundance = 75.77%; chlorine-37: atomic mass = 36.966 amu and% abundance = 24.23%.
Agilent IDP-3 Dry Pump - for 5973/5975/5977 MSD, no more oil. M) and the mass of the lighter isotope (i.e. For Cl use 35 not 35.5, or, for Br use 79 and not 80) 35 Cl: 37 Cl exists naturally in an almost 3:1 ratio, so we observe peaks at 'M' (molecules with an atom of 35 Cl) and 'M+2' (molecules an atom of 37 Cl) are obtained with relative intensity 3:1. Determine the mass of chlorine in the original sample as the difference in mass between the anhydrous sample (after drying in the crucible) and the mass of copper(1g). From the mass of chlorine, determine the moles of Cl. Determine the experimental molar ratio of Cu:Cl. In Step 12, describe the components involved in the reaction.
The atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, D). The atomic mass is a weighted average of all of the isotopes of that element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. (Atomic mass is also referred to as atomic weight, but the term 'mass' is more accurate.)
For instance, it can be determined experimentally that neon consists of three isotopes: neon-20 (with 10 protons and 10 neutrons in its nucleus) with a mass of 19.992 amu and an abundance of 90.48%, neon-21 (with 10 protons and 11 neutrons) with a mass of 20.994 amu and an abundance of 0.27%, and neon-22 (with 10 protons and 12 neutrons) with a mass of 21.991 amu and an abundance of 9.25%. The average atomic mass of neon is thus:


| 0.9048 | × | 19.992 amu | = | 18.09 amu |
| 0.0027 | × | 20.994 amu | = | 0.057 amu |
| 0.0925 | × | 21.991 amu | = | 2.03 amu |
| 20.18 amu |

The atomic mass is useful in chemistry when it is paired with the mole concept: the atomic mass of an element, measured in amu, is the same as the mass in grams of one mole of an element. Thus, since the atomic mass of iron is 55.847 amu, one mole of iron atoms would weigh 55.847 grams. The same concept can be extended to ionic compounds and molecules. One formula unit of sodium chloride (NaCl) would weigh 58.44 amu (22.98977 amu for Na + 35.453 amu for Cl), so a mole of sodium chloride would weigh 58.44 grams. One molecule of water (H2O) would weigh 18.02 amu (2×1.00797 amu for H + 15.9994 amu for O), and a mole of water molecules would weigh 18.02 grams.
Chlorine Molar Mass
The original periodic table of the elements published by Dimitri Mendeleev in 1869 arranged the elements that were known at the time in order of increasing atomic weight, since this was prior to the discovery of the nucleus and the interior structure of the atom. The modern periodic table is arranged in order of increasing atomic number instead.